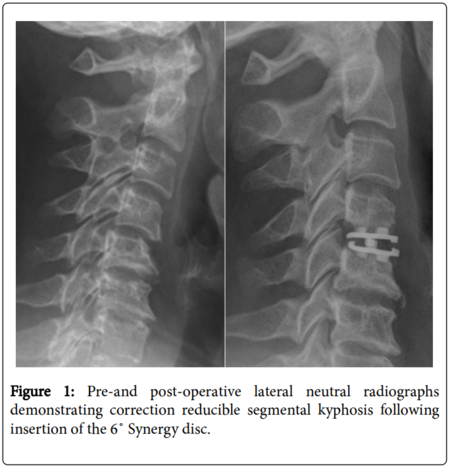
Degeneration of the cervical disc can result in loss of disc height, resulting in disc space narrowing and collapse [[[1]]]. In symptomatic, degenerated segments with parallel or kyphotic vertebral endplates, the goal of reconstruction of the disc space after decompression incorporates a strategy to recreate segmental cervical lordosis [[[2]]]. Adjacent segment disease (ASD), which is a suspected consequence of anterior cervical discectomy and fusion (ACDF), can be potentially delayed by preserving disc space kinematics of the functional spinal unit with cervical arthroplasty [[[3,4]]]. The Synergy Disc, a new cervical disc replacement, has been designed to create or preserve segmental lordosis following discectomy. Early theoretical and clinical experience suggests it has alignment advantages over existing cervical disc replacements [[[5,6]]]. The goal of the present study was to report the 2-year results of the Synergy cohort and to determine if it can provide a sagittal alignment correction comparable to the gold standard of ACDF.

