
Advancing the Standard of Care Through
Simple Design, Real Innovation™
Surgeon-Led Innovation
We are a company founded by surgeons for surgeons
with a focus on innovation to solve
unmet clinical and patient needs.
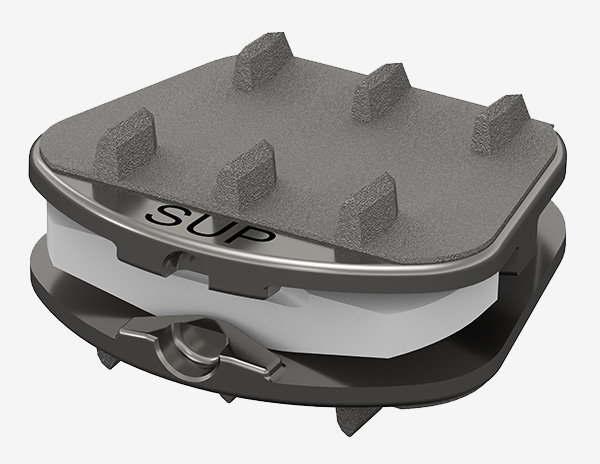
Combining Cervical Alignment & Balance with Natural Motion
Our flagship product – the Synergy Disc® – advances the standard of care for cervical artificial disc replacement devices, as it is the first disc to restore both alignment and balance while preserving natural physiologic motion.
The Synergy Disc is currently commercially available in Australia, Europe, Canada (SAP), South Africa, United Arab Emirates, Malaysia, and New Zealand. In the United States, Synergy Spine Solutions® Inc. is currently enrolling patients in its IDE study.
Latest News
Surgeon Testimonials
I have been performing total disc replacement for the treatment of cervical degenerative disc diseases since 2014 and have implanted over 300 patients during this time with varying degrees of intermediate to long term outcome. This, in large part is due to the constraints of previous artificial disc replacement designs. Most early artificial disc replacement were simple replacement designs. Most early artificial disc replacement were simple domed devices with fixed centre of rotation; this brought about unwanted constraints in the motion of the operated segments that lead to eventual prosthetic failures as well as potential hazard to the adjacent segment loading/movement and stresses that can ultimately cause pain and disability. Now with mobile core polyethylene with highly congruent endplate surfaces with lordotic options as seen in Synergy cervical disc replacements, it allows further freedom with carefully thought-out restraints to safely permit motion in more axis than before. This improved center of rotation to mimic the natural kinematics of cervical spine. I believe it is this unique set of features that make this prosthesis stand out among others and has been my choice for cervical TDR.
Dr. John Choi
The Synergy Disc procedure is intuitive and forgiving. I loved that I could see the immediate alignment correction - the importance of which I cannot overstate. This is a fantastic next generation device. (As quoted from press release 1-28-21)
Dr. Daniel Peterson
The gold standard (cervical fusion) involves proper reconstruction of sagittal balance following decompression. Conventional disc replacements, however, do just the opposite; they restore range of motion but they have an unpredictable impact on sagittal balance. The Synergy Disc combines the best of both of these very successful treatments by restoring range of motion and normal sagittal balance to the patient. Restoring alignment of the spine is vital for long-term success of cervical disc replacements. Our long-term follow-up has demonstrated outstanding results. The SYNERGY Disc will help to expand the indications for cervical arthroplasty.
Dr. Kemal Yucesoy
Neurosurgeon involved in pilot studyRestoration and maintenance of spinal alignment is critical in spine surgery. The Synergy Disc addresses correction of sagittal balance in addition to restoring physiologic motion. It has become my preferred cervical artificial disc and standard of care within my practice.
Dr. Greg Malham
Neurosurgeon. Victoria, Australia


